Core Therapeutic Areas
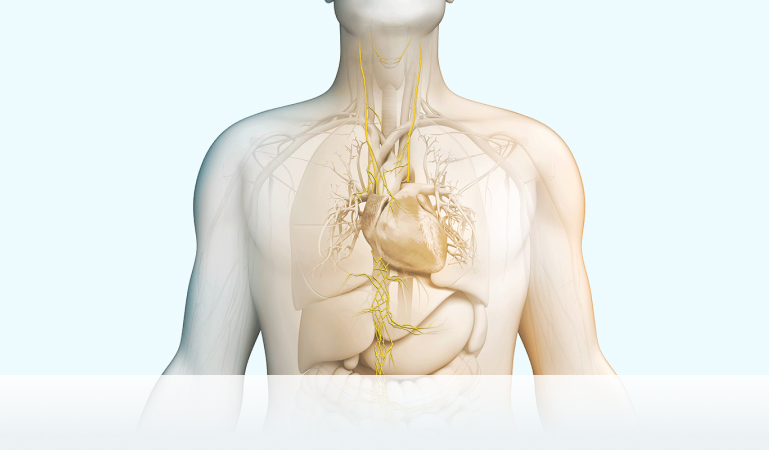
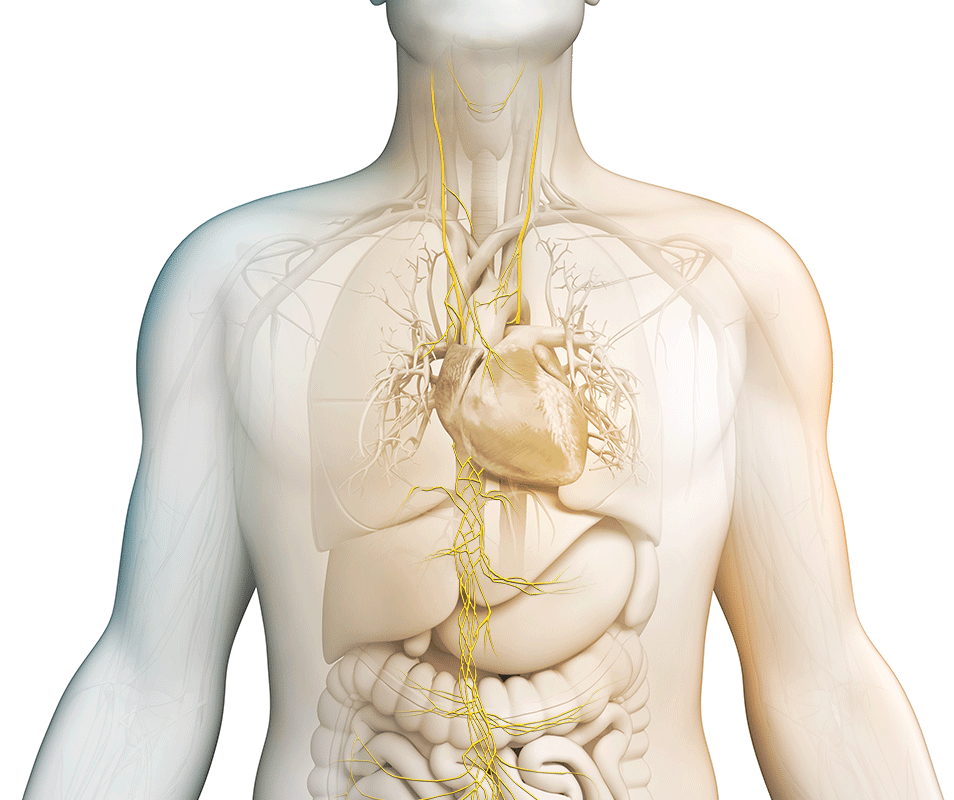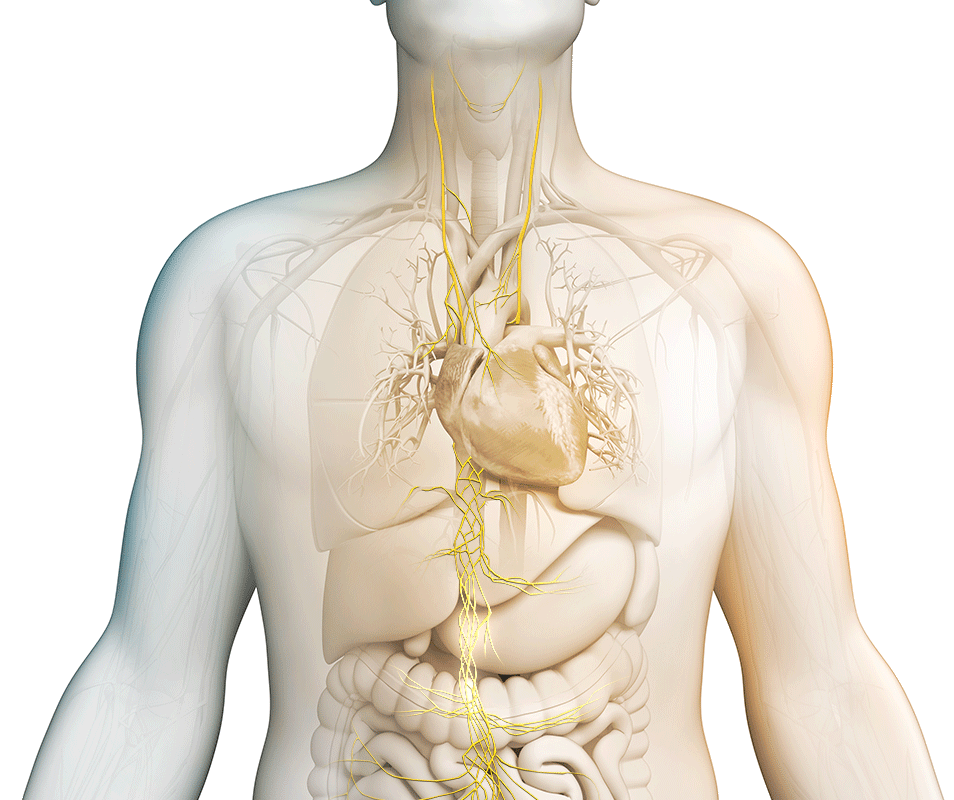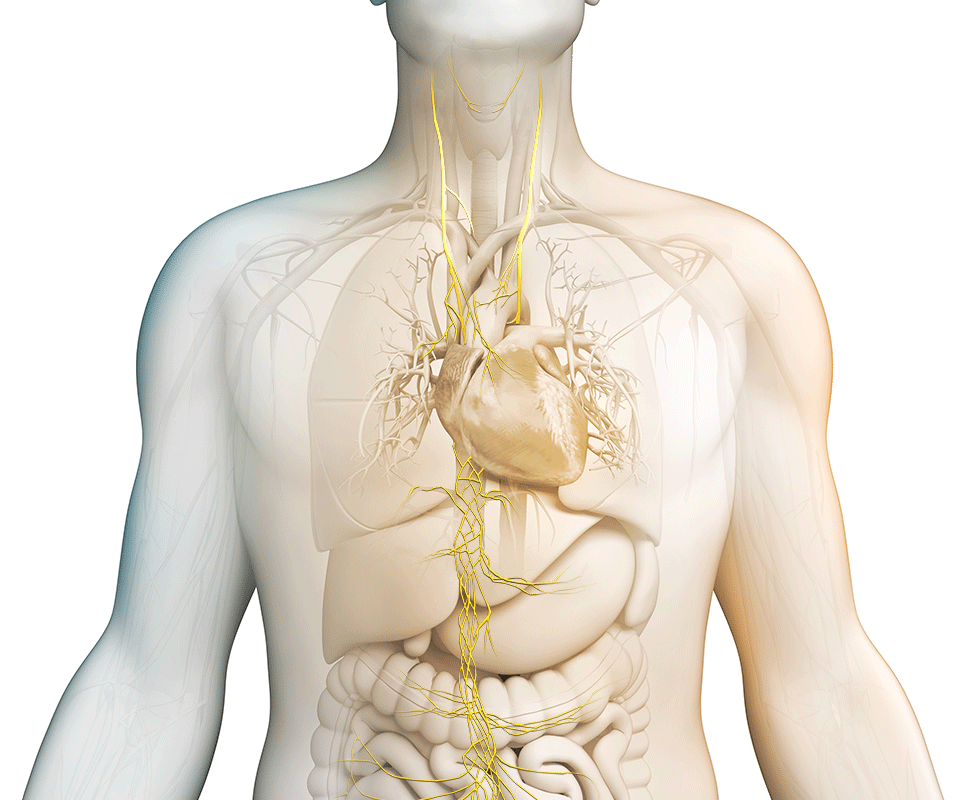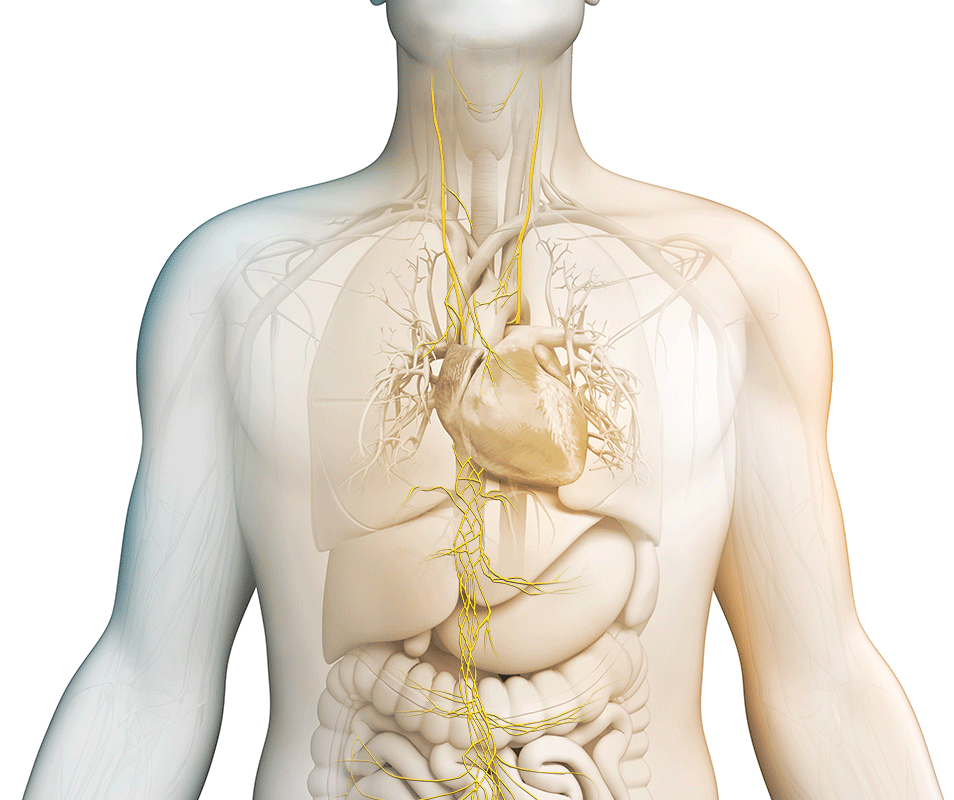
We strive to maximize the clinical potential of our innovative vagus nerve stimulation technology with research and development efforts and collaborative research partnerships. Discover how we are transforming patient care across various therapeutic areas.
Please note: Information about pipeline products or investigational uses of products does not imply FDA approval for these products or uses, nor does it establish the safety or efficacy of these products or uses. There is no guarantee that the pipeline products or investigational uses will receive FDA
approval. electroCore does not recommend or suggest use of its medicines in a manner inconsistent with FDA-approved labeling.
gammaCore™ non-invasive vagus nerve stimulator (nVNS) was granted Breakthrough Device Designation for Post-Traumatic Stress Disorder (PTSD) by the US Food and Drug Administration in January 2022. gammaCore nVNS has not been approved by FDA for use in PTSD and the safety and effectiveness of gammaCore for PTSD has not been established.
PTSD is a complex condition involving neurobiological, psychological, and physiological factors. Emerging research highlights the significant role of the vagus nerve in modulating stress responses and emotional regulation. Studies suggest that vagus nerve stimulation (VNS) could offer a therapeutic approach by targeting this crucial neural pathway, which may help alleviate symptoms and improve the overall quality of life for individuals with PTSD.1,2,3
Explore the latest Clinical Practice Guidelines, developed by the Vagus Nerve Society, for treating PTSD symptoms with nVNS. Click below to download the guidelines directly from their website.
gammaCore™ non-invasive vagus nerve stimulator (nVNS) has not been approved by FDA for use in Opioid Use Disorder (OUD) and the safety and effectiveness of gammaCore for OUD has not been established.
Opioid Use Disorder is a challenging condition characterized by compulsive opioid use despite harmful consequences, driven by complex neurobiological and psychological mechanisms. Recent studies suggest that vagus nerve stimulation (VNS) has the potential to influence neural circuits involved in addiction and withdrawal. By targeting the vagus nerve, VNS may help manage cravings and withdrawal symptoms. Integrating VNS into treatment strategies may enhance recovery outcomes and support long-term sobriety.4,5
gammaCore™ non-invasive vagus nerve stimulator (nVNS) has not been approved by the FDA for use in the treatment of gastrointestinal diseases, and the safety and effectiveness of gammaCore for gastrointestinal diseases has not been established.
Gastrointestinal diseases, including conditions like gastroparesis and irritable bowel syndrome, often involve dysregulation of the vagus nerve, impacting motility and digestion. Studies suggest that vagus nerve stimulation (VNS) could be a promising therapeutic approach, potentially enhancing gastric motility and alleviating symptoms such as nausea and abdominal pain. By modulating neural pathways, VNS may help improve gastrointestinal function, and provide an alternative approach for managing these chronic conditions, potentially improving patient outcomes and quality of life.6,7
gammaCore™ non-invasive vagus nerve stimulator (nVNS) has not been approved by the FDA for use in the treatment of Parkinson's Disease, and the safety and effectiveness of gammaCore for Parkinson's Disease has not been established.
Parkinson's Disease is a progressive neurodegenerative disorder characterized by motor symptoms such as tremors, rigidity, and bradykinesia, along with non-motor symptoms. Research indicates that vagus nerve stimulation (VNS) may influence the neural pathways involved in motor control and neuroplasticity. VNS has shown potential in alleviating both motor and non-motor symptoms by modulating brain activity and neurotransmitter release. This approach may offer a complementary treatment option that could improve the quality of life for individuals with Parkinson's Disease.8,9
gammaCore™ non-invasive vagus nerve stimulator (nVNS) has not been approved by the FDA for use in the treatment of brain injury, and the safety and effectiveness of gammaCore for brain injury has not been established.
Brain injuries lead to significant cognitive, emotional, and functional deficits. Studies suggest that vagus nerve stimulation (VNS) may promote neuroplasticity and functional recovery in individuals with brain injuries. Additionally, nVNS prophylaxis could help ameliorate neuronal dysfunction associated with brain injury in populations at high risk for concussions. This potential therapeutic approach could offer a potential adjunctive treatment to improve outcomes and quality of life for those affected by brain injuries.10,11,12
Contact Medical Affairs
Our Medical Affairs team works to inform and educate healthcare professionals about our medical devices and consumer products by providing clinical and scientific information that can support clinical decision-making. To speak to a member of the Medical Affairs team, please complete the form below.
References:
1. The Institute for Functional Medicine. (2023, December 22). Understanding PTSD from a polyvagal perspective | The Institute for Functional Medicine. https://www.ifm.org/news-insights/understanding-ptsd-from-a-polyvagal-perspective/
2. Bergland, C. (2022, January 22). Vagal nerve stimulation calms fight-or-flight responses and lowers inflammation. Psychology Today. https://www.psychologytoday.com/us/blog/the-athletes-way/202201/how-does-vagus-nerve-stimulation-reduce-ptsd-symptoms
3. Study: Vagus nerve stimulation shows progress against PTSD Symptoms. (n.d.). News Center. https://news.utdallas.edu/health-medicine/study-vagus-nerve-stimulation-shows-progress-again/
4. Lee, Y. K., Gold, M. S., Blum, K., Thanos, P. K., Hanna, C., & Fuehrlein, B. S. (2024). Opioid use disorder: current trends and potential treatments. Frontiers in Public Health, 11. https://doi.org/10.3389/fpubh.2023.1274719
5. Qureshi, I. S., Datta-Chaudhuri, T., Tracey, K. J., Pavlov, V. A., & Chen, A. C. H. (2020). Auricular neural stimulation as a new non-invasive treatment for opioid detoxification. Bioelectronic Medicine, 6(1). https://doi.org/10.1186/s42234-020-00044-6
6. Cirillo, G., Negrete-Diaz, F., Yucuma, D., Virtuoso, A., Korai, S. A., De Luca, C., Kaniusas, E., Papa, M., & Panetsos, F. (2022). Vagus nerve stimulation: a personalized therapeutic approach for Crohn’s and other inflammatory bowel diseases. Cells, 11(24), 4103.
https://doi.org/10.3390/cells11244103
7. Bonaz, B. (2023). Non-invasive vagus nerve stimulation: the future of inflammatory bowel disease treatment? Bioelectronic Medicine, 9(1). https://doi.org/10.1186/s42234-023-00129-y
8. adminVN. (n.d.). Understanding the role of the vagus nerve in Parkinson’s disease. https://vagusnerve.com/understanding-the-role-of-the-vagus-nerve-in-parkinsons-disease/
9. Figueiredo, M. (2021, June 11). Hand-held Device for Vagus Nerve Stimulation of Benefit in Parkinson’s. Parkinson’s News Today. https://parkinsonsnewstoday.com/news/electrocore-vagus-nerve-stimulation-device-eases-motor-symptoms-trial/
10. Evancho, A., Tyler, W. J., & McGregor, K. (2023). A review of combined neuromodulation and physical therapy interventions for enhanced neurorehabilitation. Frontiers in Human Neuroscience, 17. https://doi.org/10.3389/fnhum.2023.1151218
11. Wang, L., Gao, F., Wang, Z., Liang, F., Dai, Y., Wang, M., Wu, J., Chen, Y., Yan, Q., & Wang, L. (2023). Transcutaneous auricular vagus nerve stimulation in the treatment of disorders of consciousness: mechanisms and applications. Frontiers in Neuroscience, 17.
https://doi.org/10.3389/fnins.2023.1286267
12. Jin, Z., Dong, J., Wang, Y., & Liu, Y. (2023). Exploring the potential of vagus nerve stimulation in treating brain diseases: a review of immunologic benefits and neuroprotective efficacy. European Journal of Medical Research, 28(1). https://doi.org/10.1186/s40001-023-01439-2
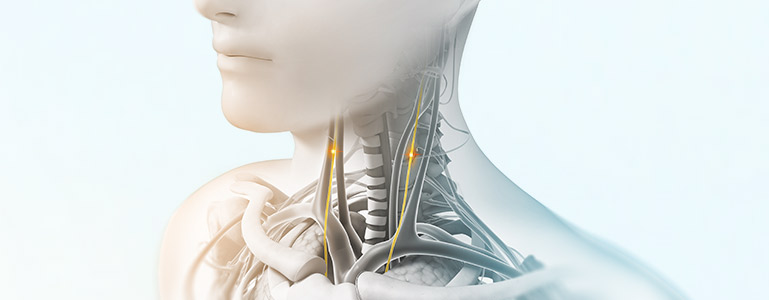
nVNS therapy
Vagus nerve stimulation is a breakthrough technology that helps control multiple disorders.

Our history
Founded on the interest in the pioneering concept of using vagus nerve stimulation to improve patient outcomes in a variety of conditions.